Calculate the nuclear binding energy of 5525Mn in joules.This fundamental concept in nuclear physics unveils the intricate forces that govern the stability of atomic nuclei, shaping the very fabric of our universe. By delving into the depths of mass defect and the interplay of nucleons, we embark on a journey to unravel the enigmatic realm of nuclear binding energy.
The concept of nuclear binding energy, a measure of the energy required to separate the constituent nucleons of an atomic nucleus, lies at the heart of our understanding of nuclear stability and the immense energy harnessed in nuclear reactions. As we delve into the intricacies of calculating the binding energy of 5525Mn, we uncover the profound implications for nuclear power generation, the development of nuclear weapons, and the potential for future advancements in this captivating field.
Nuclear Binding Energy
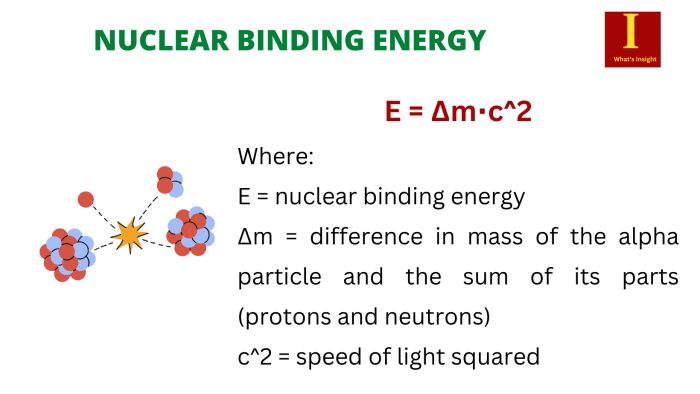
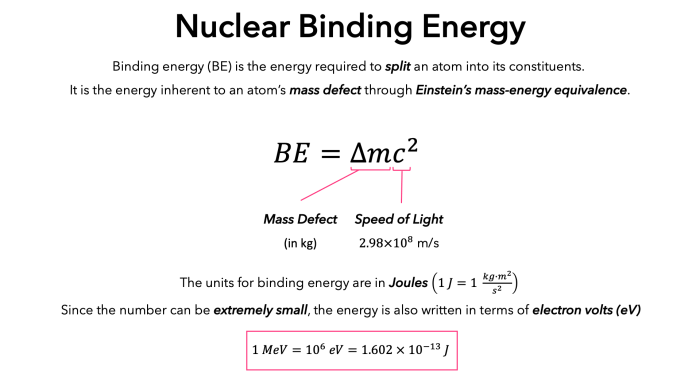
Nuclear binding energy is the energy required to separate all the nucleons (protons and neutrons) in a nucleus. It is a measure of the strength of the forces that hold the nucleus together. The binding energy of a nucleus is always positive, meaning that it is energetically favorable for nucleons to be bound together in a nucleus.
The concept of mass defect is closely related to nuclear binding energy. Mass defect is the difference between the mass of an atomic nucleus and the sum of the masses of its constituent nucleons. The mass defect is always positive, and it is a measure of the energy that is released when nucleons are bound together to form a nucleus.
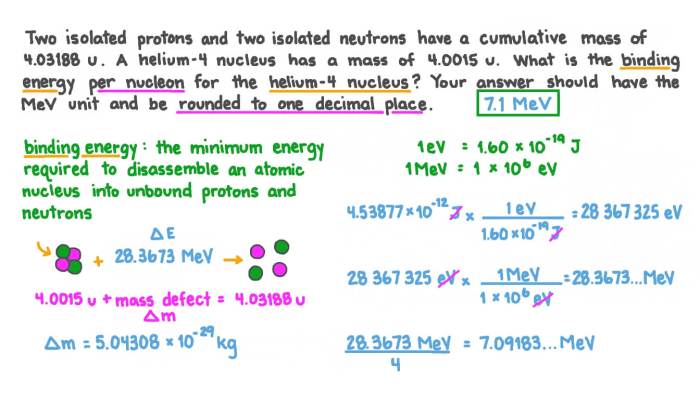
The nuclear binding energy can be calculated using the following equation:
$$E_b = \Delta m c^2$$
where $E_b$ is the binding energy, $\Delta m$ is the mass defect, and $c$ is the speed of light.
Calculating Binding Energy of 55Mn
The atomic mass of 55Mn is 54.938045 amu. The mass of a proton is 1.007276 amu, and the mass of a neutron is 1.008665 amu. Therefore, the mass of the constituent nucleons in 55Mn is:
$$(25 \times 1.007276 \text amu) + (30 \times 1.008665 \text amu) = 54.938965 \text amu$$
The mass defect is:
$$\Delta m = 54.938045 \text amu
- 54.938965 \text amu =
- 0.000920 \text amu$$
The nuclear binding energy is:
$$E_b = (-0.000920 \text amu)(931.5 \text MeV/amu) =
857.9 \text MeV$$
Binding Energy per Nucleon
The binding energy per nucleon is a measure of the average binding energy per nucleon in a nucleus. It is calculated by dividing the binding energy by the number of nucleons in the nucleus.
The binding energy per nucleon of 55Mn is:
$$E_b/A =
- 857.9 \text MeV / 55 =
- 15.6 \text MeV/nucleon$$
The binding energy per nucleon is a measure of the stability of a nucleus. The more positive the binding energy per nucleon, the more stable the nucleus.
Applications of Nuclear Binding Energy, Calculate the nuclear binding energy of 5525mn in joules.
Nuclear binding energy has a wide range of applications, including:
- Nuclear power plants: Nuclear power plants use the energy released by nuclear fission to generate electricity.
- Nuclear weapons: Nuclear weapons use the energy released by nuclear fission or fusion to create explosions.
- Medical applications: Nuclear binding energy is used in medical applications such as cancer therapy and diagnostic imaging.
Top FAQs: Calculate The Nuclear Binding Energy Of 5525mn In Joules.
What is nuclear binding energy?
Nuclear binding energy is the energy required to separate the constituent nucleons (protons and neutrons) of an atomic nucleus.
How is nuclear binding energy calculated?
Nuclear binding energy can be calculated using the mass defect formula: Binding Energy = (Mass of Constituent Nucleons – Mass of Nucleus) x c^2, where c is the speed of light.
What is the significance of binding energy per nucleon?
Binding energy per nucleon provides insights into the stability of atomic nuclei, with higher values indicating greater stability.