As the ethical implications of citi conflicts of interest in human subjects research come under scrutiny, this comprehensive analysis delves into the complexities of this topic. Citi’s role as an institutional review board (IRB) and the potential conflicts of interest that may arise are examined, providing a critical perspective on the impact of these conflicts on research ethics.
This in-depth exploration unravels the strategies employed by Citi to mitigate potential conflicts of interest, ensuring the objectivity and integrity of the IRB review process. Case studies are analyzed to shed light on specific instances where Citi’s IRB has been accused of conflicts of interest, highlighting the lessons learned and implications for the broader research community.
1. Citi’s Role in Human Subjects Research
Citi is a commercial organization that provides a range of services to the healthcare industry, including institutional review board (IRB) services. IRBs are independent bodies that review and oversee human subjects research to ensure that it is conducted ethically and in accordance with applicable regulations.
Citi’s IRB services are used by a wide range of institutions, including universities, hospitals, and research centers.
As an IRB, Citi has a number of responsibilities and obligations. These include:
- Reviewing and approving research protocols to ensure that they meet ethical and regulatory standards.
- Monitoring ongoing research to ensure that it is being conducted in accordance with the approved protocol.
- Responding to any complaints or concerns about the ethical conduct of research.
Citi has a long history of providing IRB services. The company has been accredited by the Association for the Accreditation of Human Research Protection Programs (AAHRPP) since 1998. Citi’s IRB services have been used to review and oversee a wide range of research studies, including clinical trials, observational studies, and social and behavioral research.
2. Potential Conflicts of Interest: Citi Conflicts Of Interest In Human Subjects Research
There are a number of potential conflicts of interest that may arise in Citi’s role as an IRB. These include:
- Financial conflicts of interest:Citi is a for-profit company, and it may have a financial incentive to approve research studies that are likely to generate revenue for the company. This could lead to a conflict of interest if the IRB is reviewing a study that is being conducted by a company that is a client of Citi.
- Institutional conflicts of interest:Citi provides IRB services to a wide range of institutions. This could lead to a conflict of interest if the IRB is reviewing a study that is being conducted by one of Citi’s clients. The IRB may be reluctant to disapprove a study that is being conducted by a client, even if the study has ethical concerns.
- Personal conflicts of interest:Members of the IRB may have personal relationships with researchers who are conducting studies that are being reviewed by the IRB. This could lead to a conflict of interest if the IRB member is biased in favor of the researcher or the study.
These are just a few of the potential conflicts of interest that may arise in Citi’s role as an IRB. It is important to note that not all conflicts of interest are necessarily unethical. However, it is important to be aware of potential conflicts of interest and to take steps to mitigate them.
3. Mitigation Strategies
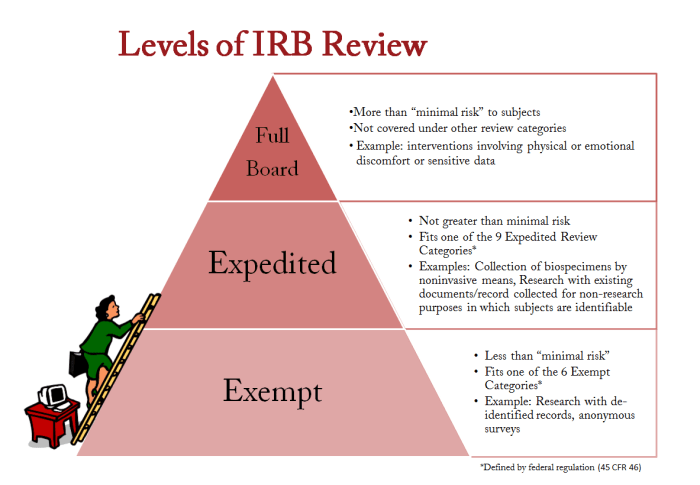
Citi has implemented a number of strategies to mitigate potential conflicts of interest. These include:
- Disclosure of conflicts of interest:All members of the IRB are required to disclose any potential conflicts of interest. This information is then reviewed by the IRB chair, who makes a determination as to whether the conflict of interest is significant enough to warrant the IRB member being recused from the review of the study.
- Recusal of IRB members:IRB members who have a significant conflict of interest are recused from the review of the study. This means that they do not participate in the discussion or vote on the study.
- Independent review:In some cases, Citi may hire an independent reviewer to review a study that has a potential conflict of interest. The independent reviewer is not affiliated with Citi or the institution that is conducting the study. This helps to ensure that the review is objective and unbiased.
These are just a few of the strategies that Citi has implemented to mitigate potential conflicts of interest. By implementing these strategies, Citi helps to ensure that the IRB review process is objective and unbiased.
Quick FAQs
What is the role of Citi in human subjects research?
Citi serves as an institutional review board (IRB), responsible for the ethical review and oversight of human subjects research.
How can conflicts of interest arise in Citi’s role as an IRB?
Conflicts of interest may arise when Citi has financial or other ties to researchers or institutions involved in the research being reviewed.
What strategies has Citi implemented to mitigate potential conflicts of interest?
Citi has implemented strategies such as conflict of interest disclosure policies, training for IRB members, and independent review of research proposals.