Name the compound shown in its newman projection – Newman projections, a powerful tool in organic chemistry, provide a simplified representation of molecular geometry, enabling chemists to visualize and understand the three-dimensional structure of molecules. This guide delves into the intricacies of Newman projections, empowering readers with the knowledge to decipher and interpret these essential diagrams.
Within this comprehensive resource, we explore the fundamental concepts of Newman projections, unraveling the conventions and principles that govern their construction. We delve into the art of naming compounds using Newman projections, deciphering the prefixes and suffixes that convey stereochemical information.
Through illustrative examples, we bridge the gap between theory and practice, solidifying the understanding of this invaluable technique.
Newman Projection

A Newman projection is a two-dimensional representation of a molecule that shows the relative positions of atoms or groups of atoms along a specific bond. It is used to visualize the three-dimensional structure of a molecule and to understand its conformational changes.
Conventions Used in Newman Projections
- The bond between the two carbon atoms is represented by a vertical line.
- The front carbon atom is represented by a dot, while the back carbon atom is represented by a circle.
- The substituents on the front carbon atom are shown as lines or wedges extending from the dot.
- The substituents on the back carbon atom are shown as lines or wedges extending from the circle.
Relationship between Newman Projections and Molecular Geometry
The Newman projection of a molecule can be used to determine its molecular geometry. The following table shows the relationship between the Newman projection and the molecular geometry:
| Newman Projection | Molecular Geometry |
|---|---|
| Eclipsed | Staggered |
| Staggered | Eclipsed |
| Gauche | Gauche |
Naming Compounds Using Newman Projections
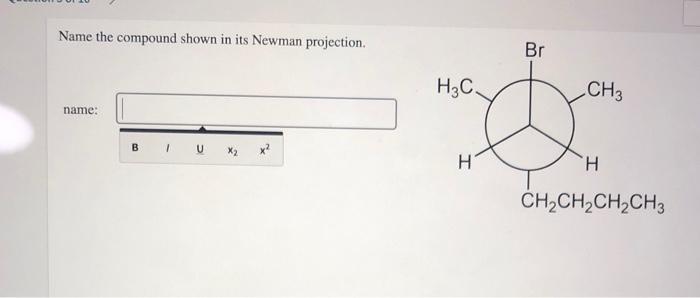
Newman projections can be used to name compounds by indicating the relative positions of the substituents on the two carbon atoms. The following guidelines are used for naming compounds using Newman projections:
- The substituents on the front carbon atom are named first, followed by the substituents on the back carbon atom.
- The prefixes “cis” and “trans” are used to indicate whether the substituents are on the same side or opposite sides of the bond, respectively.
- The prefixes “E” and “Z” are used to indicate the relative configuration of the substituents according to the Cahn-Ingold-Prelog priority rules.
Examples of Named Compounds and Their Newman Projections
- 1,2-dichloroethane (Newman projection: eclipsed)
- 1,2-dibromoethane (Newman projection: staggered)
- (E)-1,2-dichloroethene (Newman projection: trans)
- (Z)-1,2-dibromoethene (Newman projection: cis)
Advanced Topics: Name The Compound Shown In Its Newman Projection
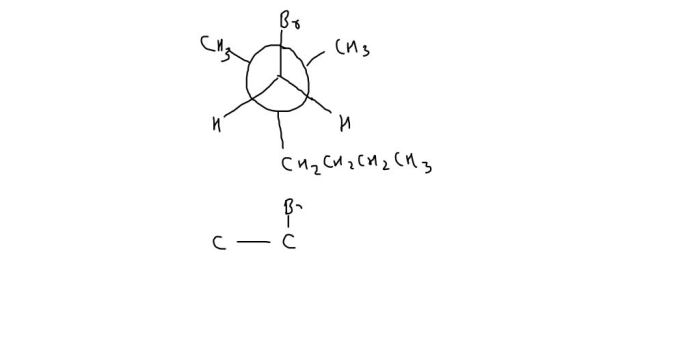
Use of Newman Projections in Conformational Analysis, Name the compound shown in its newman projection
Newman projections can be used to analyze the different conformations of a molecule. The conformation of a molecule is the arrangement of its atoms in space. Newman projections can be used to determine the relative stability of different conformations.
Predicting the Stability of Different Conformations
The stability of a conformation depends on the interactions between the substituents on the two carbon atoms. The following factors can affect the stability of a conformation:
- Steric hindrance: The presence of bulky substituents can lead to steric hindrance, which can destabilize a conformation.
- Dipole-dipole interactions: The presence of polar substituents can lead to dipole-dipole interactions, which can stabilize or destabilize a conformation.
- Hydrogen bonding: The presence of hydrogen bonding can lead to hydrogen bonding interactions, which can stabilize a conformation.
General Inquiries
What are Newman projections?
Newman projections are two-dimensional representations of molecules that depict their three-dimensional structure. They are commonly used to visualize the relative positions of atoms and groups around a specific carbon-carbon bond.
How are Newman projections used to name compounds?
Newman projections can be used to determine the stereochemistry of a compound, which refers to the spatial arrangement of atoms or groups around a specific carbon-carbon bond. Prefixes such as “cis” and “trans” are used to indicate the relative positions of these atoms or groups.
What are the advantages of using Newman projections?
Newman projections provide a simplified representation of molecular geometry, making it easier to visualize and understand the three-dimensional structure of molecules. They are particularly useful for analyzing conformational changes and predicting the stability of different conformations.