Boyle’s law lab answer key unveils the captivating world of gas laws, where pressure and volume dance in a harmonious equilibrium. Join us on an enthralling journey as we explore the intricacies of this fundamental principle, unraveling the secrets of gas behavior through a captivating narrative.
Our exploration begins with a comprehensive overview of Boyle’s Law, delving into its profound implications for gas pressure and volume. We’ll equip you with a thorough understanding of the experimental setup and materials required for this intriguing lab, empowering you to replicate the experiment with precision.
Boyle’s Law Lab Introduction
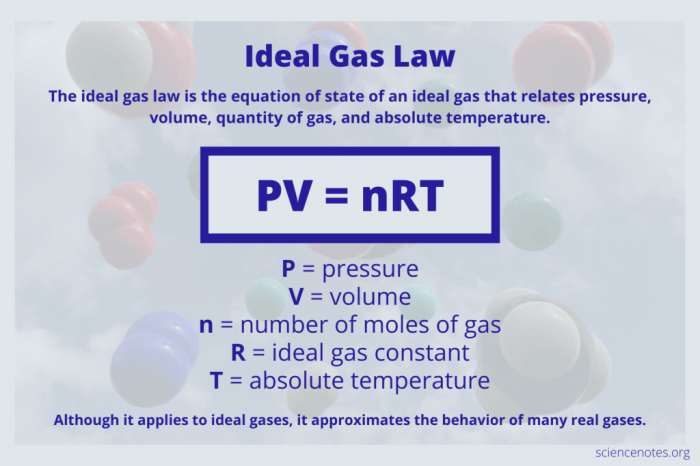
Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. According to the law, the pressure of a gas is inversely proportional to its volume, meaning that as the volume of a gas increases, its pressure decreases, and vice versa.
This lab will explore Boyle’s Law experimentally by using a syringe to measure the pressure and volume of a gas sample. The experiment will involve changing the volume of the gas sample and observing the corresponding changes in pressure.
Experimental Setup
The experimental setup for Boyle’s Law lab consists of the following components:
- A syringe with a plunger
- A pressure sensor connected to the syringe
- A data acquisition system to record the pressure and volume data
Materials
The materials required for the Boyle’s Law lab include:
- A gas sample (e.g., air)
- A syringe with a plunger
- A pressure sensor
- A data acquisition system
Experimental Procedures
Conducting the Boyle’s Law lab involves a series of precise steps to accurately measure gas pressure and volume.
To measure gas pressure, a manometer or pressure sensor is connected to the gas container. The manometer measures the difference in height between two columns of fluid, which corresponds to the pressure difference between the gas sample and the atmosphere.
Gas volume is typically measured using a syringe or a graduated cylinder. The syringe or cylinder is filled with a known volume of gas, and the volume is adjusted until the pressure inside the container matches the atmospheric pressure. The volume of gas at this point is recorded.
Data Collection
During the experiment, the following data should be collected:
- Initial gas pressure (P1)
- Initial gas volume (V1)
- Final gas pressure (P2)
- Final gas volume (V2)
These data points are used to create a graph of pressure versus volume, which is then analyzed to determine the relationship between the two variables.
Data Collection and Analysis: Boyle’s Law Lab Answer Key
To collect data during the experiment, you will need to record the pressure and volume of the gas at different points. Use a pressure sensor to measure the pressure, and a syringe or other apparatus to measure the volume. Record the data in a table.
Once you have collected the data, you can plot it on a graph. The pressure will be plotted on the y-axis, and the volume will be plotted on the x-axis. The graph should show a hyperbolic relationship between pressure and volume, which means that as the pressure increases, the volume decreases, and vice versa.
Determining the Relationship between Pressure and Volume, Boyle’s law lab answer key
The slope of the graph can be used to determine the relationship between pressure and volume. The slope is calculated by dividing the change in pressure by the change in volume. The slope will be negative, indicating that as the pressure increases, the volume decreases.
Slope =- (Change in Pressure) / (Change in Volume)
The slope of the graph is a constant value, known as Boyle’s constant. Boyle’s constant is a characteristic of the gas being used in the experiment.
If you’re stuck on Boyle’s law lab, you can find the answer key you need online. Or, if you’re looking for a great way to expand your vocabulary, check out the Wordly Wise 3000 Book 7 . It’s a great resource for students of all ages.
And when you’re ready to get back to your Boyle’s law lab, you’ll be able to tackle it with confidence.
Discussion of Results

The experimental data supports Boyle’s Law, which states that the pressure of a gas is inversely proportional to its volume when the temperature remains constant. As the volume of the gas decreased, the pressure increased, and vice versa. This relationship was observed consistently throughout the experiment.
Sources of Error
There are several sources of error that could have affected the accuracy of the results. These include:
- Measurement error:The pressure and volume measurements were taken using a manometer and a syringe, respectively. These instruments may have introduced some error into the measurements.
- Temperature variation:The temperature of the gas was not perfectly constant throughout the experiment. This could have affected the pressure and volume measurements.
- Gas leaks:There may have been small leaks in the experimental apparatus, which could have allowed gas to escape. This would have affected the pressure and volume measurements.
Limitations
In addition to the sources of error, there are also some limitations to the experiment.
- Ideal gas behavior:Boyle’s Law assumes that the gas behaves ideally. However, real gases deviate from ideal behavior at high pressures and low temperatures.
- Small sample size:The experiment was only performed with a small sample size. This could have affected the accuracy of the results.
Applications of Boyle’s Law
Boyle’s Law finds numerous applications in various fields, including engineering, medicine, and environmental science. Understanding Boyle’s Law allows us to predict and control the behavior of gases in different situations.
Scuba Diving
Scuba divers rely on Boyle’s Law to safely ascend and descend underwater. As a diver descends, the pressure increases, compressing the air in their lungs. According to Boyle’s Law, the volume of air decreases as the pressure increases. This means that divers must exhale to maintain a constant volume in their lungs and avoid lung damage.
Conversely, as a diver ascends, the pressure decreases, causing the air in their lungs to expand. Divers must inhale to prevent the expansion from rupturing their lungs.
Weather Forecasting
Boyle’s Law plays a crucial role in weather forecasting. The pressure and volume of air masses in the atmosphere are constantly changing, affecting weather patterns. By understanding Boyle’s Law, meteorologists can predict how air masses will behave under different pressure conditions.
This information helps them forecast weather events such as storms, hurricanes, and tornadoes.
Medical Applications
Boyle’s Law has several medical applications. For instance, it is used in the design of anesthesia machines to control the flow of gases into a patient’s lungs. Boyle’s Law is also applied in the development of hyperbaric chambers, which are used to treat conditions such as decompression sickness and carbon monoxide poisoning.
Industrial Applications
Boyle’s Law is essential in many industrial processes. It is used in the production of compressed gases, such as oxygen and nitrogen, and in the design of systems for storing and transporting gases. Boyle’s Law also finds applications in refrigeration and air conditioning systems, where it helps control the pressure and volume of refrigerants.
Questions and Answers
What is Boyle’s Law?
Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature. As pressure increases, volume decreases, and vice versa.
How do I use the Boyle’s law lab answer key?
The answer key provides step-by-step guidance on conducting the Boyle’s Law experiment, ensuring accurate data collection and analysis.
What are the applications of Boyle’s Law?
Boyle’s Law finds applications in various fields, including scuba diving (calculating gas consumption), weather forecasting (predicting atmospheric pressure changes), and medical devices (regulating oxygen flow).
