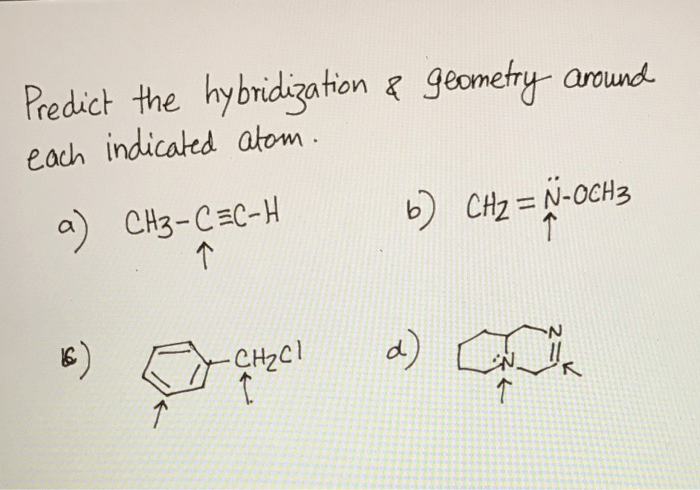
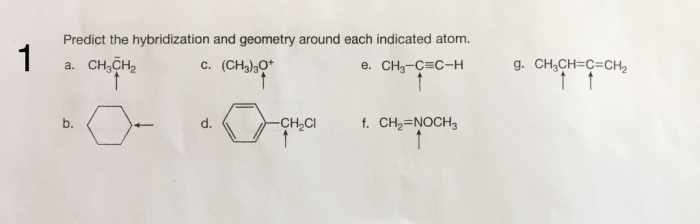
Predict the hybridization and geometry around each indicated atom – Predicting hybridization and geometry around each indicated atom is a crucial aspect of understanding molecular structure and properties. This guide delves into the concepts of hybridization and molecular geometry, providing a comprehensive overview of the methods used to predict these characteristics.
Through engaging discussions and practical examples, we will explore the applications of hybridization and geometry predictions in various fields of chemistry.
Hybridization of Indicated Atom: Predict The Hybridization And Geometry Around Each Indicated Atom
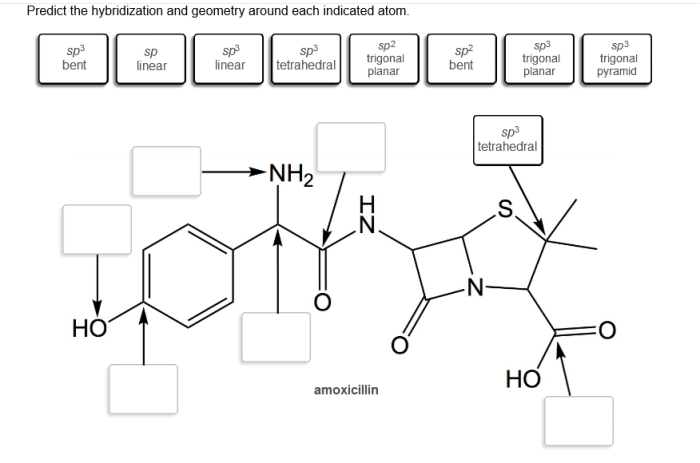
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies. This concept is crucial in understanding the molecular geometry and properties of compounds.
To predict the hybridization of an indicated atom, we must first identify its valence electrons. Valence electrons are the electrons in the outermost energy level of an atom, and they participate in chemical bonding. The number of electron pairs around the indicated atom, including both bonding and lone pairs, determines the hybridization.
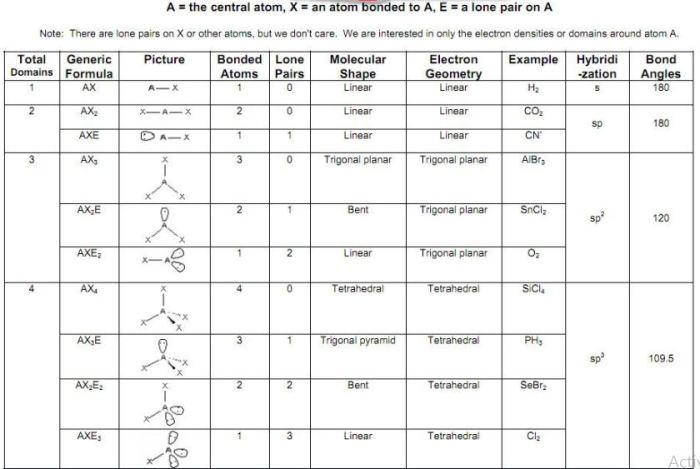
The VSEPR (Valence Shell Electron Pair Repulsion) theory provides a useful tool for predicting the hybridization of an atom based on the number of electron pairs around it.
Geometry Around Indicated Atom
The hybridization of an atom directly influences the molecular geometry around it. Different types of hybridization result in specific molecular shapes, such as:
- sp hybridization: linear geometry
- sp 2hybridization: trigonal planar geometry
- sp 3hybridization: tetrahedral geometry
By predicting the hybridization of an indicated atom, we can also predict the molecular geometry around it.
Examples of Hybridization and Geometry
| Atom | Hybridization | Molecular Geometry | Explanation |
|---|---|---|---|
| CH4 | sp3 | Tetrahedral | Four electron pairs (four C-H bonds) around the central carbon atom result in sp3 hybridization and a tetrahedral molecular geometry. |
| BF3 | sp2 | Trigonal Planar | Three electron pairs (three B-F bonds) around the central boron atom result in sp2 hybridization and a trigonal planar molecular geometry. |
| H2O | sp3 | Bent | Two electron pairs (two O-H bonds) and two lone pairs around the central oxygen atom result in sp3 hybridization, but the presence of lone pairs distorts the geometry from tetrahedral to bent. |
| CO2 | sp | Linear | Two electron pairs (two C-O bonds) around the central carbon atom result in sp hybridization and a linear molecular geometry. |
Methods for Predicting Hybridization and Geometry, Predict the hybridization and geometry around each indicated atom
The VSEPR theory is the most commonly used method for predicting hybridization and geometry. It is based on the principle that electron pairs around an atom will repel each other and adopt an arrangement that minimizes this repulsion.
Other methods include:
- Molecular orbital theory
- Density functional theory
Applications of Hybridization and Geometry Predictions
Predicting hybridization and geometry is essential in understanding molecular structure and properties. It has applications in various fields, including:
- Chemistry
- Biochemistry
- Materials science
By understanding the hybridization and geometry of molecules, scientists can gain insights into their reactivity, bonding, and overall behavior.
General Inquiries
What is hybridization?
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies.
How do you predict the hybridization of an atom?
To predict the hybridization of an atom, you need to determine the number of valence electrons and the number of electron pairs around the atom.
What is the relationship between hybridization and molecular geometry?
The hybridization of an atom determines the shape of the electron cloud around the atom, which in turn determines the molecular geometry.